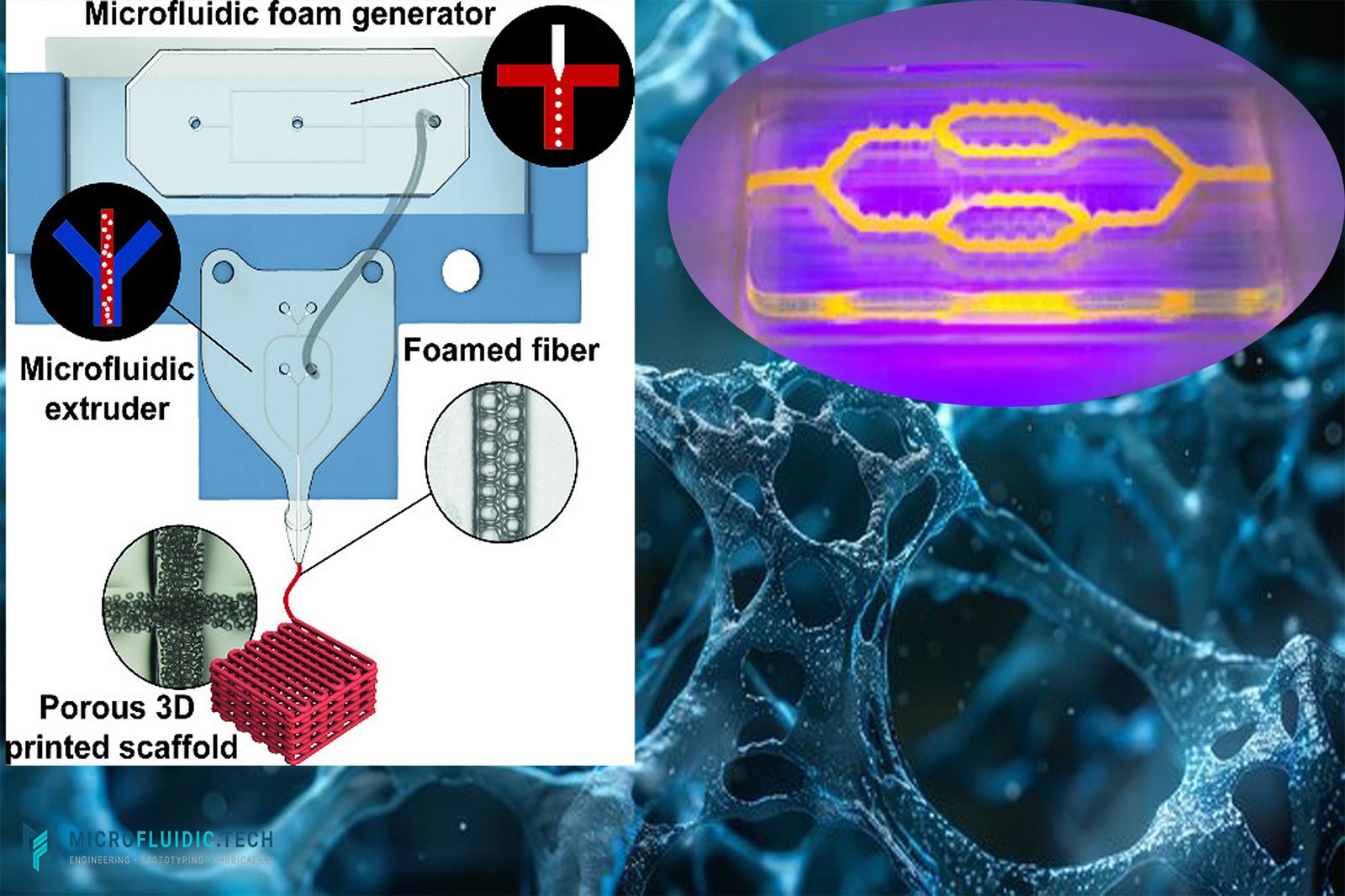
The rapid rise of microfluidics has reshaped the landscape of material engineering and biomedical research. By enabling precise control over fluids and cells at microscale levels, microfluidic technology has opened new doors in diagnostics, drug delivery, and regenerative medicine. Among its most exciting frontiers lies microfluidic biomaterials—an interdisciplinary field that merges microfluidics with biomaterials science to create smarter, more adaptive biomedical tools.
From Silicon to Smart Biomaterials
Microfluidic systems have evolved significantly over the last two decades. Early platforms were fabricated using silicon and glass, prized for their precision but limited in flexibility and scalability. The shift to polymers brought cost-effectiveness, ease of prototyping, and broader accessibility. More recently, researchers have turned to paper-based platforms, valued for their portability and potential in low-resource settings.
This material progression underscores a central theme in microfluidics: adaptability. Each generation of materials reflects efforts to balance precision with practicality, moving closer to real-world clinical applications.
Organ-on-a-Chip and the Role of 3D Bioprinting
One of the most transformative applications of microfluidics is the organ-on-a-chip (OoAC) platform. By mimicking the structural and functional properties of human tissues, OoAC devices allow researchers to study disease progression, evaluate drug responses, and reduce reliance on animal testing.
Here, 3D bioprinting plays a critical role. Unlike conventional fabrication techniques, bioprinting enables the integration of living cells, extracellular matrix components, and bioactive molecules into microfluidic chips with high precision. This capability allows for the design of more physiologically relevant models—whether recreating lung alveoli, vascular networks, or tumor microenvironments.
Biomaterials in Drug Delivery, Cell Culture, and Tissue Engineering
Microfluidic biomaterials extend far beyond organ-on-a-chip devices. Their applications include:
-
Drug Delivery: Microfluidic systems enable controlled release of therapeutics, improving targeting while minimizing side effects.
-
Cell Culture: Microengineered scaffolds provide cells with dynamic environments that better reflect in vivo conditions.
-
Tissue Engineering: Microfluidic biomaterials guide the growth of complex tissues, offering a pathway toward regenerative solutions for damaged organs.
These applications highlight the potential of microfluidics to reshape not only laboratory research but also the future of personalized medicine.
Challenges and Future Directions
Despite remarkable progress, several challenges remain:
-
Scalability: Transitioning prototypes into mass-producible devices remains difficult.
-
Stability: Ensuring long-term performance of biomaterials under physiological conditions is a persistent obstacle.
-
Clinical Translation: Bridging the gap between lab innovation and clinical adoption requires regulatory clarity and validation in real-world settings.
Looking ahead, the future of microfluidic biomaterials will likely involve:
-
Hybrid Fabrication Approaches: Combining conventional molds with bioprinted elements for scalable yet customizable devices.
-
AI Integration: Leveraging machine learning to monitor drug release, predict tissue responses, and guide personalized therapies in real time.
-
Interdisciplinary Collaboration: Advancing this field will require coordinated efforts across materials science, micromachining, and clinical medicine.
Conclusion
Microfluidic biomaterials represent a new era of innovation at the intersection of engineering and medicine. By merging the precision of microfluidics with the adaptability of biomaterials and the versatility of 3D bioprinting, researchers are laying the groundwork for breakthroughs in drug screening, tissue engineering, and personalized healthcare.
The path forward is clear: hybrid designs, AI-driven monitoring, and deep interdisciplinary partnerships will be key to moving organ-on-a-chip platforms from the lab bench into the clinic—transforming patient care in the process
Source: